The term VOC (Volatile Organic Compound) refers to a wide range of carbon-containing chemical compounds. At room temperature, VOCs tend to be liquid or solid, with a high vapour pressure that means they readily vaporise into gaseous states. In recent years, increasing attention has been paid to VOCs as pollutants and health hazards. This blog will provide an introduction to VOCs and their effects on human health and the environment, and give an overview of regulations on VOC emission.
Sources of VOC in air
VOC exist naturally in the atmosphere through processes including vegetation growth and soil activity, as well as in biomass burning. However, a significant portion of VOC build-up in the atmosphere come from man-made sources; these include emissions from road traffic and from chemical processes such as crude oil cracking; and products that emit high concentrations of VOCs (for example, paint, solvents and varnishes).
Effects of VOCs on human health
The effects of VOC emissions on human health varies widely according to context. Some VOCs are harmless, but many are toxic at low levels, while others are flammable at higher concentrations. Repeated and long-term, low-level exposure to harmful VOCs can cause serious health issues. For example, formaldehyde, styrene, benzene and other aromatics are known carcinogens – thus, exposure to traffic exhaust fumes, smoking and strong solvents can present serious health risks. Growing awareness of the chronic toxicity of VOCs has led to reduced occupational exposure limits (OEL) and increased requirements for direct measurement.
You can read more about the dangers of VOCs in our white paper.

Environmental issues caused by VOCs
Aside from adverse health implications, the emission of VOCs into the atmosphere causes serious environmental issues. One of these is the formation of ground-level ozone, which leads to smog.
Normally, ozone (or O3) is naturally formed at high altitude in the atmosphere (stratosphere) when O2 molecules are separated into individual oxygen particles by UV radiation. These free oxygen particles then collide with other O2 molecules to become ozone. While we know that ozone helps to protect our planet from the sun’s harmful UV rays, tropospheric, or ground-level, ozone is not emitted directly into the air, but is created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs).
Here is an example of how nitrogen dioxide can lead to the creation of ozone:
NO2 + Sunlight (UV rays) = NO + O
The free oxygen particle then attaches itself to an O2 molecule and becomes ozone. This happens when pollutants emitted by vehicles, power plants, chemical plants and other sources chemically react in the presence of sunlight. Breathing ozone can cause respiratory irritation and may exacerbate respiratory diseases such as bronchitis and asthma. Ozone at ground-level is hazardous to plants and negatively affects crops.
In addition to ground-level ozone formation, some VOCs may cause odour problems, due to their high odour intensity. Processes like waste incineration, food processing and wastewater treatment emit lots of malodorous gases and are often subject to odour nuisance complaints from nearby residents. Foul smelling gases including H2S, NH3 and VOCs are a significant problem for many industries, including the pharmaceutical, food and beverage, textile and tannery sectors.
At high concentrations, foul-smelling VOCs can cause dizziness and respiratory issues. For this reason, regulations are in place to reduce VOCs in ambient air.
Directives and regulations on VOC emission
Most countries have directives and regulations on VOC emission. In Europe, the relevant directives on atmospheric pollutants, including VOC emissions, are 2001/81/EC and 2016/2284. The first directive sets national emissions ceilings for the VOC emission from all sources, which were to be reached by the year 2010. The second directive specifies the percentage reduction in VOC emissions, both for the individual country and the EU as an entire area.
In addition to outdoor VOC emissions, many countries have implemented regulations to limit the use of VOCs in consumer products. In the EU, Directive 2004/42/EC specifies emission limits for VOCs, prompted by the use of organic solvents in decorative paints and varnishes and in vehicle refinishing products. The directive sets the maximum permissible contents of VOCs in g/L. The directive also requires that suppliers label the subcategory of the product, defines the legal limit value for VOC contents and gives the maximum content of VOC permissible for the product in its ready-to-use condition.
In our next blog we will discuss Crowcon’s unique solution for VOC detection in ambient air.
References
1. Odor-causing volatile organic compounds in wastewater treatment plant units and sludge management areas (J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008 Nov)
2. Ground-level ozone basics (US Environmental Protection Agency guide)
3. Do volatile organic compounds smell? (Foobot website)
4. Volatile organic compounds (VOC) and consumer products regulations (Chem Safety Pro website)
5. Effective and sustainable VOC removal with ozone and AOP (Ozonetech website)

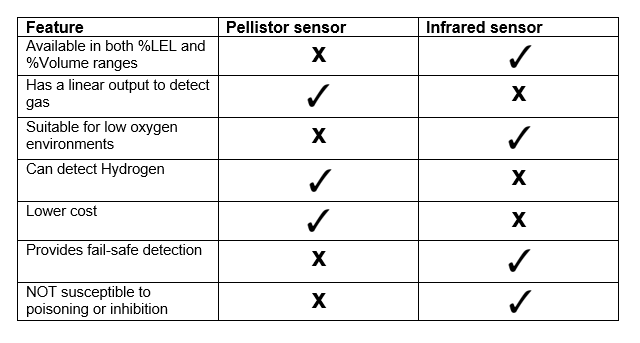
 H2 gas has a high propensity to leak due to its very small size and its low density (0.09 g/L at NTP of 0°C / 1 atm) which corresponds to a high buoyancy.
H2 gas has a high propensity to leak due to its very small size and its low density (0.09 g/L at NTP of 0°C / 1 atm) which corresponds to a high buoyancy. Not only does leakage decrease process efficiency, but it becomes a serious safety concern. Hydrogen has a Lower Explosive Limit (LEL) of just 4% volume, meaning even tiny quantities of H2 can cause explosions when mixed with atmospheric air. Even a spark of static electricity from a person’s finger is enough to trigger an explosion when hydrogen is present.
Not only does leakage decrease process efficiency, but it becomes a serious safety concern. Hydrogen has a Lower Explosive Limit (LEL) of just 4% volume, meaning even tiny quantities of H2 can cause explosions when mixed with atmospheric air. Even a spark of static electricity from a person’s finger is enough to trigger an explosion when hydrogen is present.






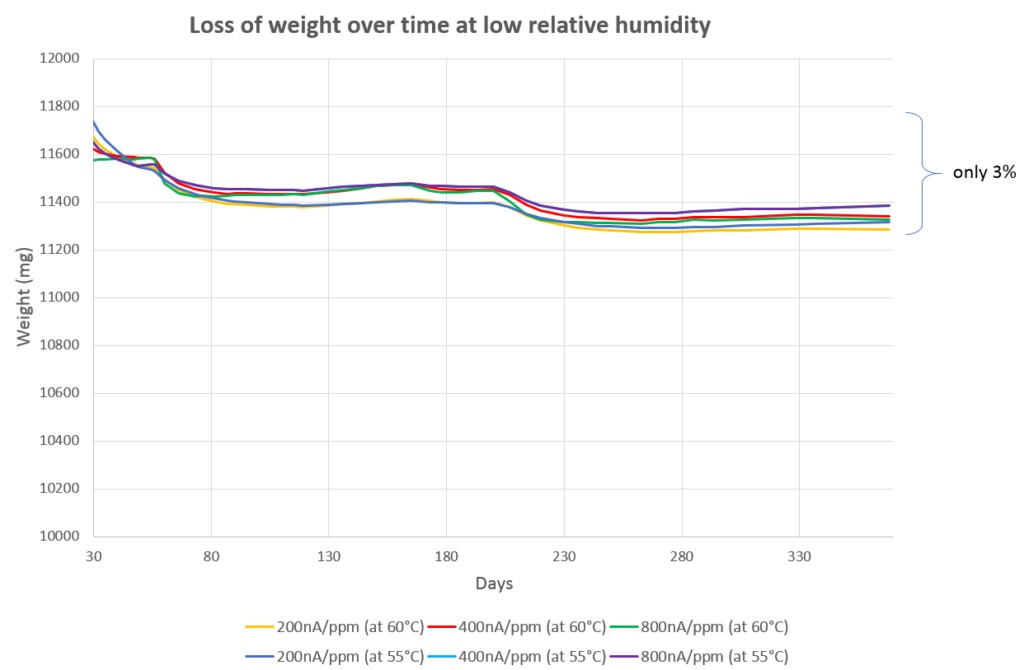
 In traditional H2S sensors, detection is based on electrochemical technology, where electrodes are used to detect changes induced in an electrolyte by the presence of the target gas. However, high temperatures combined with low humidity causes the electrolyte to dry out, impairing sensor performance so that the sensor has to be replaced regularly; meaning high replacement costs, time and efforts.
In traditional H2S sensors, detection is based on electrochemical technology, where electrodes are used to detect changes induced in an electrolyte by the presence of the target gas. However, high temperatures combined with low humidity causes the electrolyte to dry out, impairing sensor performance so that the sensor has to be replaced regularly; meaning high replacement costs, time and efforts.